How effective will it be to opt for Rapid Diagnostic Test ?
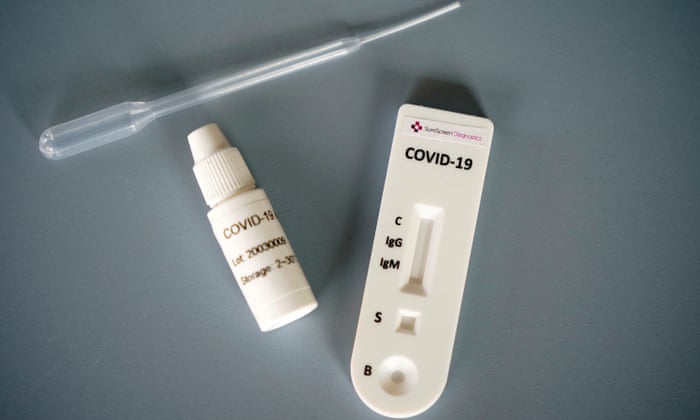
The country has already entered the second stage of COVID-19 following the recent confirmation of the first locally transmitted COVID-19 case. Taking into consideration the possible mass outbreak of the disease, the government has enrolled large-scale rapid diagnostic test (RDT) throughout the country.
However, the underlying concern is on exactness of the result and the quality of the RDT kit. There is no diagnostic test that produce 100% valid result. Still, real time Reverse Transcription Polymerase Chain Reaction (RT-PCR) is regarded as the most standard test for diagnosis of COVID-19 which requires at least require Biosafety Level-2 (BSL-2) or equivalent laboratory facilities and qualified laboratory technologist. As of now, laboratory diagnosis of COVID-19 is being carried in only in 15 different laboratories of Nepal including central laboratory in Kathmandu- National Public Health Laboratory (NPHL). It has been learnt around 500 and 3,000 tests are carried out every day through RT-PCR and RDT Kit respectively in Nepal. There are many instances that test positives from RDT turned negative when verified with RT-PCR.
Dr. Khem Karki, Health Advisor to the Minister of Health and Population on 31st March announced the government has restricted the use of RDT of COVID-19 stating the WHO has not recommended the use of rapid test and the kits are not reliable for diagnosis of the disease. However, he defended the Government’s move on procurement of the RDTs from China stating that the kit would be in use during epidemic outbreak of COVID-19 in the country. Dr Karki even claimed that the kits will be brought into operation only after assessing its quality and reliability by the Nepal Health Research Council (NHRC).
Contrary to its such declaration, the government meanwhile has carried rapid test in name of early diagnosis through out country in wake of confirmation of first community transmission and looming epidemic threat of COVID-19.
As the global outbreak of COVID-19 outbreak has put lives of country’s entire population at threat and, questions being raised over quality of the RDT kits brought from China, the government before opting for the large scale testing must disclose quality of the tests through measures of validity which are sensitivity, specificity and predictive values of the test. It is realized that even the government personnel are more concerned on reliability of the test than validity. Reliability refers producing consistent result by a diagnostic kit under similar circumstances whereas validity is defined as ability of the test to measures what it claims to measure. Validity of the kit is simply the accuracy on identification of the disease. Even if the test kit is reliable, the result produced from the same kit may not be necessarily accurate or valid. However, the valid results are basically reliable: if the kit produces accurate result, the result should be reproducible under numerous trails under similar conditions. Therefore if a test is not valid it is absolutely absurd on discussing over its reliability.
RDT is typically a laterally flow assay that is small, portable, easy to perform, non-automated and can be used as a point of care (POC). The test result can be obtained within 30 minutes after blood sample is subjected to the kit. RDT identifies antibodies against the SARS-CoV-2 virus namely IgM and IgG in the blood of infected person. In the early stage of infection typically after 3-7 days of symptoms appearance the antibodies may be below the detection limit of the kit. Therefore the test cannot accurately diagnose acute infection to rule out or rule in the presence of the infection. Besides, the COVID-19 infected person with a reduced ability to fight against infections like patients of diabetes, HIV/AIDS, genetic disorders, malnutrition or other immunocompromised person may result false negative-that is, the kit showing that a person is not infected when they are infected. In such backdrop, it will not be effective to carry RDT to confirm the presence of virus in a person in early stage of infection and for early diagnosis of the disease. However, the RDT with acceptable level of validity can be used alone as a screening tool.
It is evident that public have divided opinions regarding the government’s decision to conduct RDT for COVID-19 diagnosis. The government moreover has been slammed for its decision to bring WHO non-certified kit into use for test. Interestingly, the government has not made public yet the verdict of NHRC and NPHL over quality of the RDT kits. The criticism has intensified as the WHO Nepal Office stated that RDT kits are not reliable for conducting RDT test.
It would be a wise decision for deploying RDT kit if the kit has an acceptable level of quality and the erroneous result produced through the test has low risk to the suspects and their contacts. In name of early diagnosis of COVID-19 cases, it will be unethical to trail RDT with unknown parameters of accuracy of the test. Meanwhile, it’s too early side to subject its population to RDT test by the government as it requires significant level of outbreak of disease in the country. Besides, the Government must develop rapid test protocol guidelines that confirms negative test results through RT-PCR. Until confirmed by RT-PCR, every negative should be placed in quarantine or isolation under medical surveillance to observe the prognosis of disease and to prevent the possible transmission. Every test should be simultaneously carried with counseling and referrals for treatment in one visit.
The other need of this hour is the government must install more 96-Well PCR Thermal Cyclers across the country and further conduct coordination with private laboratories and hospitals to utilize their RT-PCR. To meet current demand of large scale diagnosis trained lab technologists working in private sector and academia must deployed. In resource-poor setting like ours, the investigation of the disease should be carried out based on clinical and epidemiological evidence, contact tracing or other active case finding mechanism.
Hyperlinks:
https://apps.who.int/iris/rest/bitstreams/1272454/retrieve
https://english.khabarhub.com/2020/31/86199/
https://www.spotlightnepal.com/2020/04/02/nepal-bars-using-rapid-test-kits-recently-imported-china/
https://epaper.nagariknetwork.com/republica/src/epaper.php?id=3562




_rcn1YzUXpY.jpg)




Leave Comment