Maiden Pharmaceuticals: India investigates cough syrups after Gambia deaths
The Indian government has ordered an investigation into four cough syrups after the World Health Organization (WHO) linked them to the deaths of 66 children in The Gambia.
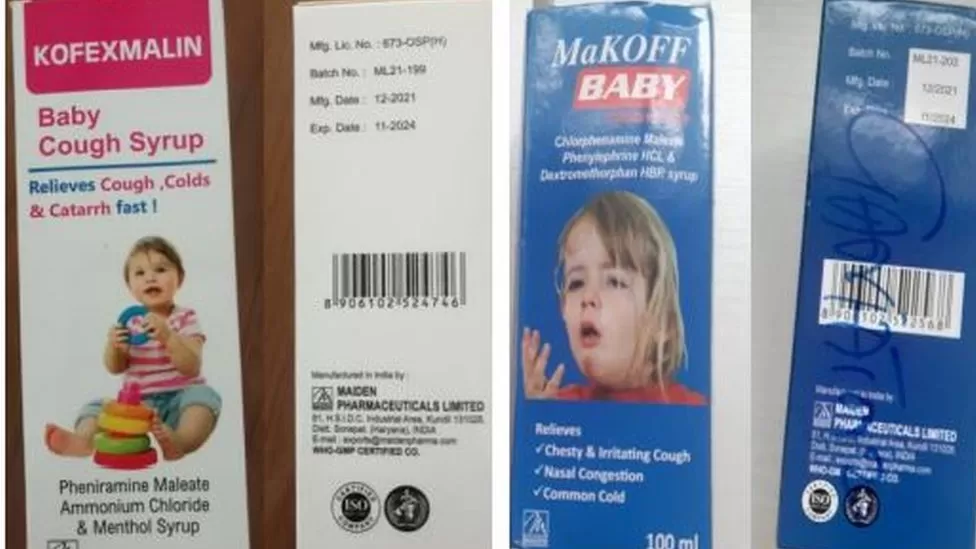
Oct 8: The WHO said the children had died of kidney injuries. It said it found "unacceptable" levels of toxins in the syrups made by Maiden Pharmaceuticals.
It also advised regulators to stop sale of the products.
Maiden Pharmaceuticals has not yet commented.
The BBC has contacted the company for comment.
India's national drug regulator began investigating after the WHO contacted it on 29 September, an Indian government statement said.
The regulator has also asked the WHO to share its report establishing the "causal relation to death with the medical products in question", the statement added.
The WHO findings, announced by Director-General Tedros Adhanom Ghebreyesus on Wednesday, came after samples of each of the four cough syrups were tested. It identified the medicines as Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
The health body said that laboratory analysis had confirmed that the syrups contained unacceptable amounts of diethylene glycol and ethylene glycol, which were toxic to humans and could prove fatal when consumed.
The WHO said that so far, the products have been identified in The Gambia, but that they may have been distributed to other countries through informal markets.
The Indian government, however, said it had exported these cough syrups "only to The Gambia so far".
The health body said it was "conducting further investigation" with the firm and Indian authorities.
"All batches of these products should be considered unsafe until they can be analysed by the relevant National Regulatory Authorities," it added.
India produces a third of the world's medicines, mostly in the form of generic drugs.
Home to some of the fastest growing pharmaceutical companies, the country is known as the "world's pharmacy" and meets much of the medical needs of African nations.
Maiden Pharmaceuticals, which is based in the northern state of Haryana, exports its products to countries in Asia, Africa and Latin America, according to Reuters.
Medical officers in The Gambia first raised the alarm in July after dozens of children were diagnosed with serious kidney problems.
The Gambia's director of health services, Mustapha Bittaye, told Reuters that the number of deaths had gone down in recent weeks and that the country had banned the sale of the products.
"However, until recently, some of the syrups were still being sold in private clinics and in hospitals," he was quoted as saying.









Leave Comment